Transforming colorectal cancer management with tumor informed ctDNA testing
Helping clinicians answer important clinical questions in the adjuvant and surveillance settings
By Dr. Adham Jurdi, MD
Circulating tumor DNA (ctDNA) has long been established as a highly prognostic biomarker to identify molecular residual disease (MRD) in colorectal cancer (CRC) as well as other solid tumor cancers1. With several MRD-guided trials underway exploring the many ways this technology can improve patient care, coupled with the expanding body of data generated over the last several years supporting tumor-informed MRD testing, clinicians are keen to learn more how to use this technology to help inform treatment decisions today. Listen to the podcast and read the article here below.
In this episode, Dr. Ruchika Talwar, a urologic oncologist at Vanderbilt University Medical Center, Dr. Adham Jurdi, a medical oncologist and Senior Director of Oncology at Natera, delve into the groundbreaking world of tumor-informed molecular residual disease testing. Discover how circulating tumor DNA (ctDNA) is revolutionizing cancer detection and treatment, providing real-time insights that surpass traditional imaging methods. Learn about the decades-long journey to perfect this technology, its clinical implications, and the exciting advancements on the horizon.

Dr. Adham Jurdi is a Board-certified Medical Oncologist who specializes in gastrointestinal malignancies. Prior to joining Natera, Dr. Jurdi was the Medical Director at Austin Cancer Center and SUNY Upstate Medical University Cancer Center, as well as being a staff oncologist at the Syracuse VAMC. Throughout his clinical career, he was involved in GI oncology and precision medicine research. He also launched several multidisciplinary tumor boards, including Austin's first Molecular Tumor Board.
ctDNA testing can offer clarity when clinical or pathological features don’t tell the whole story
Currently clinicians rely on several variables—including patient age, tumor size, lymph node involvement as well as other high risk features—to determine which patients may benefit from adjuvant chemotherapy. Because these features alone can be unreliable at predicting which patients are at high risk for relapse, physicians often end up inadvertently overtreating patients and undertreating others. This is where a more sensitive and specific tool like ctDNA testing for MRD detection can help physicians right-size treatment and inform next steps.
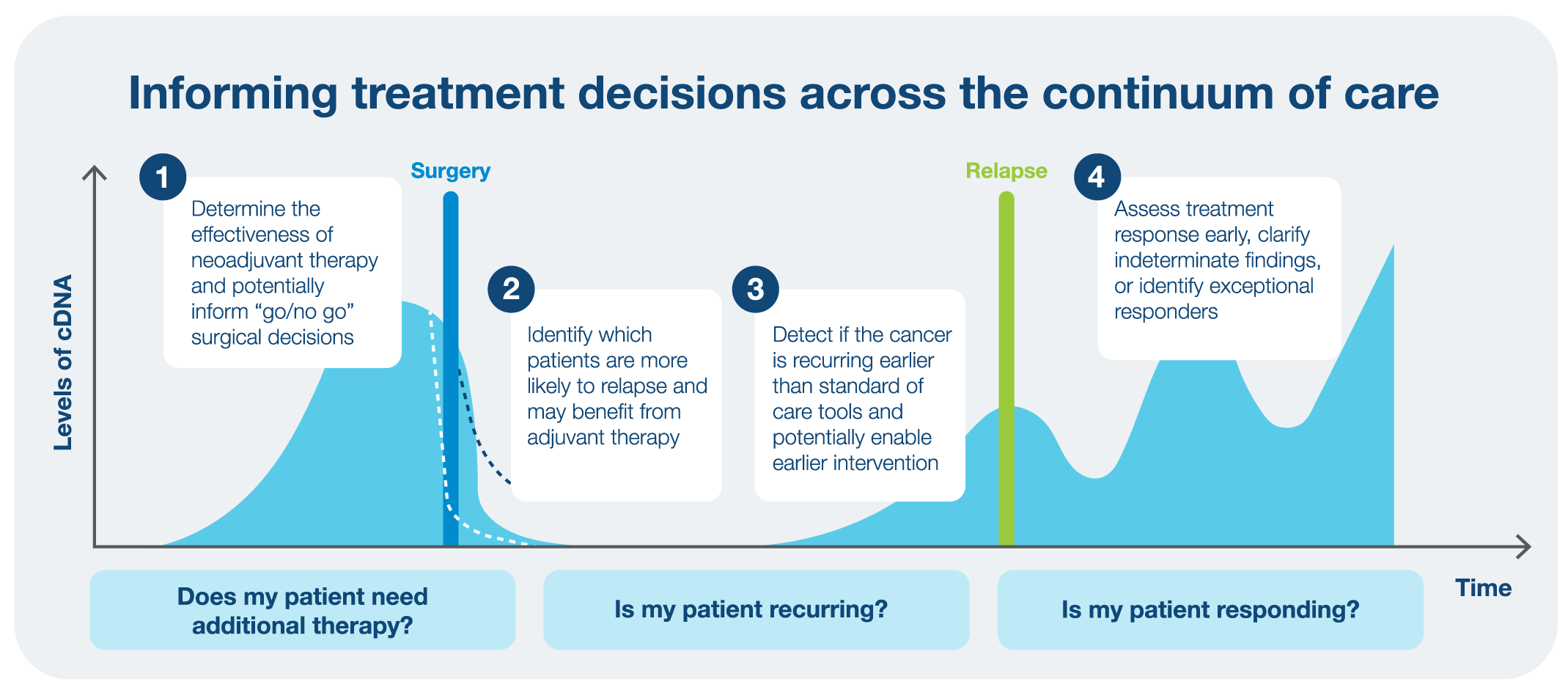
Using ctDNA to select which patients will benefit from escalated treatment
The recent read out of the Phase III CALGB (Alliance)/SWOG 80702 study2 used tumor-informed Signatera MRD testing to determine the benefit of adding celecoxib to standard of care adjuvant chemotherapy FOLFOX for nearly 1,000 stage III colon cancer. In this ad hoc analysis, patients who were Signatera-positive after surgery had a significant disease-free survival (DFS) and overall survival (OS) benefit when celecoxib was added to the adjuvant FOLFOX therapy. The addition of celecoxib significantly improved DFS in comparison to placebo with a three-year DFS of 41 percent versus 26.6 percent. For patients who were Signatera-negative, the addition of celecoxib demonstrated no survival benefit. This further reinforces what has been observed in previous data: patients who are MRD-negative and remain serially negative experience better survival than patients who are MRD-positive.
Impact of MRD testing in the adjuvant and surveillance settings
In the GALAXY 36-month analysis3 and in the recently presented final analysis of the BESPOKE CRC study4, it was found that tumor-informed MRD testing with Signatera was highly prognostic of recurrence, consistent what has been shown before: patients who were ctDNA-positive had a significant increased risk for relapse.3,4 Conversely patients who were ctDNA-negative derived no significant benefit from adjuvant chemotherapy. The BESPOKE CRC trial evaluated the ability of Signatera to inform adjuvant chemotherapy decisions for patients with Stage II/III CRC. This read out also demonstrated that longitudinal ctDNA monitoring can predict cancer recurrence in CRC with one out of six clinicians in the trial using Signatera results to inform adjustment to treatment. It was also shown that Signatera results added reassurance for the treatment plan. Of the 188 patients with disease recurrence, 30 percent received oligometastasis-directed therapy, demonstrating the actionability of tumor-informed ctDNA testing, changing the way Stage II and III CRC are managed and potentially enabling a second chance at a cure.
The future of ctDNA in colorectal cancer
The future is bright for ctDNA testing, which is expected to become more widely adopted as a standard of care tool to help inform treatment in both adjuvant and surveillance settings. Many physicians today have already begun integrating this useful tool within the clinic to enable more informed adjuvant treatment decisions, closer surveillance, and real-time treatment response monitoring, potentially enabling earlier therapeutic interventions and ultimately improving patient outcomes.
References:
1. Reinert T, et al., Analysis of Plasma Cell-Free DNA by Ultradeep Sequencing in Patients With Stages I to III Colorectal Cancer. JAMA Oncol. 2019 Aug 1;5(8):1124-1131. doi: 10.1001/jamaoncol.2019.0528. Erratum in: JAMA Oncol. 2019 Aug 1;5(8):1232
2. Nakamura, Y., Watanabe, J., Akazawa, N. et al. ctDNA-based molecular residual disease and survival in resectable colorectal cancer. Nat Med 30, 3272–3283 (2024)
3. Shaw et al., Circulating tumor DNA for Detection of Molecular Residual Disease (MRD) in Patients with Stage II/III Colorectal Cancer (CRC): Final Analysis of the BESPOKE CRC Sub cohort, as presented at presented at the 2025 American Society of Clinical Oncology Gastrointestinal Cancers Symposium (ASCO GI)
4. Shah, et al. Circulating tumor DNA for Detection of Molecular Residual Disease (MRD) in Patients with Stage II/III Colorectal Cancer (CRC): Final Analysis of the BESPOKE CRC Sub cohort, as presented at presented at the 2025 American Society of Clinical Oncology Gastrointestinal Cancers Symposium (ASCO GI)
Signatera has been developed and its performance characteristics determined by the CLIA-certified laboratory performing the test. The test has not been cleared or approved by the US Food and Drug Administration (FDA). CAP accredited, ISO 13485 certified, and CLIA certified. ©2025 Natera, Inc. All Rights Reserved