Oncologists Get Personalized, Actionable Therapeutic Insights Fast With the Comprehensive OncoExTra® Test
By Christy Russell, MD - Vice President of Medical Affairs at Exact Sciences
As an oncologist who has cared for thousands of patients with cancer, I know that spending time with them is the top priority. Every day, oncologists are busy learning patient histories, getting to know patients and their caregivers, explaining diagnoses, making treatment decisions, writing prescriptions, and helping patients through therapy—work that requires all our knowledge, skills, and empathy. When there’s time to sit at a desk and check incoming test results, it’s necessary to get a quick grasp on the essential information and use it to plan therapy.

In this third episode of the OncoExTra® podcast series, host Ruchika Talwar, MD, talks with Christy Russell, MD, Vice President of Medical Affairs at Exact Sciences, about what oncologists can expect when they receive their OncoExTra comprehensive genomic profiling test results, and how they can utilize the report and tap into Exact Sciences' skilled support team to develop a plan of action for their patients.
These priorities are always on my mind in my work with the OncoExTra® comprehensive genomic profiling test for patients with advanced solid tumors. Before joining Exact Sciences, I had already used the Oncotype DX Breast Recurrence Score® test many times to get personalized genomic insights for my patients with early-stage breast cancer. It’s exciting to me that we now also have a single test that’s innovative, comprehensive, and highly precise in its diagnostic capabilities for a range of cancers. At a glance, the report offers busy oncologists the guidance they need for personalized treatment strategies, turning unknowns into answers.
Testing that’s high tech and high touch
As oncologists, we always want the latest technology for our patients, but we also appreciate simplicity, clarity, and knowledgeable customer support. When oncologists receive the concise, actionable report of the OncoExTra test results, it may be reassuring to know the combination of advanced technology and high-touch professional involvement that goes into those results.
The OncoExTra test sequences nearly 20,000 genes1, detects biomarkers, and flags those biomarkers for which current therapies or clinical trials exist. Critically, it sequences DNA and RNA at once, allowing oncologists to efficiently employ the growing list of FDA-approved drugs that target fusions with markedly improved response rates and increased time to progression compared to standard chemotherapy.2
By incorporating a unique form of germline subtraction called patient-matched tumor-normal (PMTN) sequencing, the OncoExTra test offers exceptional accuracy in isolating somatic, targetable biomarkers and in limiting false positives. Using a patient’s tumor sample and a blood sample, the OncoExTra test sequences DNA in both, and then subtracts the normal DNA in the blood from the tumor DNA. The result is the most accurate identification of somatic variants available,3 which allows the test to find at least one clinically actionable variant in 84% of cases.1 PMTN sequencing also helps oncologists identify candidates for immune checkpoint inhibitors by delivering the gold-standard calculation of tumor mutational burden (TMB).1
We take a high-touch approach throughout the high-tech testing process. It starts the moment the specimen arrives in the lab, when a pathologist evaluates the quantity of tumor available. If the specimen contains enough tumor (20% or more of the specimen) for a reliable test, the team can quickly lock in a testing timeline. When a tumor sample is not sufficient, we ensure that oncologists are notified early in the testing process.
Once sequencing is complete, a pathologist from the Exact Sciences curation team reviews the report. They verify that it’s consistent with the patient’s specific cancer, and they validate the biomarker—drug connections and evidence associated with each finding. Finally, an Exact Sciences pathologist signs off on every report. With this combination of advanced technology and knowledgeable customer support, we deliver thorough, accurate, and actionable reports into doctors’ hands with speed and efficiency.
A report focused on treatment options
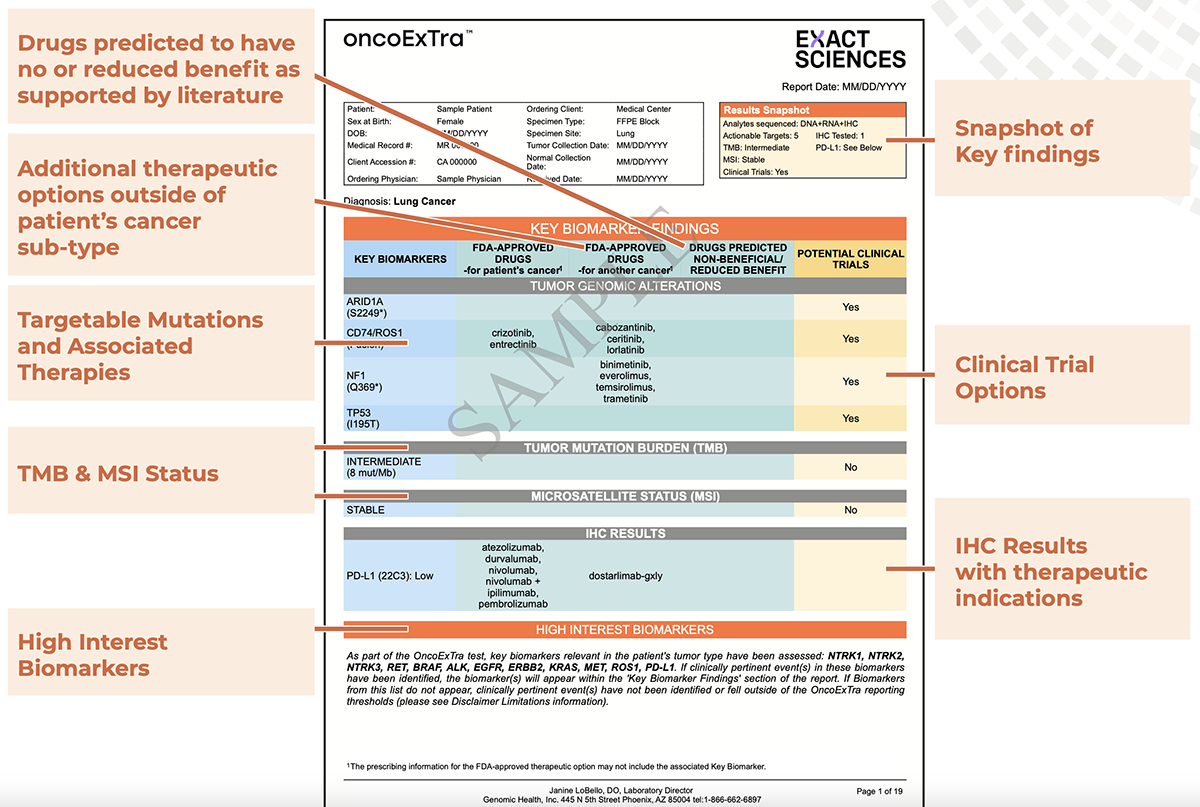
Advanced cancer is an emotionally devastating diagnosis, leaving both patients and physicians eager to initiate therapy. Testing needs to be swift and accurate. Within 14 days after Exact Sciences receives a patient’s tumor and blood samples,1 the oncologist gets a report that’s easy to read, with personalized, immediately actionable genomic insights. On the first-page summary, oncologists find all they need to know about therapies that target the patient’s key biomarkers.
The report’s front page includes a Results Snapshot with a quick overview of analytes (DNA, RNA, and proteins via optional immunohistochemistry [IHC]), number of actionable targets identified, TMB (low, intermediate, high), microsatellite status (MSI), clinical trials (Yes/No), and the IHC tests performed, including programmed death-ligand 1 (PD-L1).
Treatment options associated with each of the key biomarker findings make up the rest of the page, including TMB, MSI, and IHC results. For biomarkers in each category, the OncoExTra test identifies three potential options: 1) an FDA-approved drug for patients with the same cancer, 2) an FDA-approved drug for the same biomarker in patients with another type of cancer, and 3) clinical trials that are actively recruiting patients with that biomarker. It also lists drugs that the literature shows have a negative or have limited effect in patients with the same biomarkers.
Finally, a section called High Interest Biomarkers lists all the variants relevant to the patient’s tumor type that have targeted therapies. It explains that if a variant isn’t listed in the findings, it’s not abnormal in this patient. Although oncologists generally don’t need data on abnormalities that are not associated with a therapy or a clinical trial, all other abnormalities are listed on the remaining pages for reference.
Support from Exact Sciences experts
When oncologists have the OncoExTra test results in hand and they’re preparing to discuss the options with their patients, they sometimes have questions or want support. At Exact Sciences, we welcome all doctors to call our team of oncologists and pathologists. We make these calls a priority, making every effort to return them the same business day to meet the urgency that doctors and their patients feel to initiate a new therapy for advanced cancer. To us, this personal support goes hand in hand with comprehensive, highly accurate testing, because it allows us to help oncologists help their patients.
1. White T, Szelinger S, LoBello J, et al. Analytic validation and clinical utilization of the comprehensive genomic profiling test, GEM ExTra®. Oncotarget. 2021;12(8):726-739.
2. Zhou C, Solomon B, Loong HH, et al. First-line selpercatinib or chemotherapy and pembrolizumab in RET fusion-positive NSCLC. N Engl J Med. 2023;389(20):1839-1850.
3. Asmann YW, Parikh K, Bergsagel PL, et al. Inflation of tumor mutation burden by tumor-only sequencing in under-represented groups. NPJ Precis Oncol. 2021;5(1):22.
Christy Russell, MD, is Vice President of Medical Affairs at Exact Sciences. A medical oncologist, Dr Russell is a former director of the Norris Breast Cancer Center at the University of Southern California.
The OncoExTra test was developed, and the performance characteristics validated by Genomic Health, Inc., a wholly owned subsidiary of Exact Sciences Corporation following College of American Pathologists (CAP) and Clinical Laboratory Improvement Amendments (CLIA) regulations. The OncoExTra test is performed at the Genomic Health Phoenix clinical laboratory. Exact Sciences clinical laboratories are accredited by CAP, certified under CLIA regulations, and qualified to perform high-complexity clinical laboratory testing. This test has not been cleared or approved by the US Food and Drug Administration or other notified regulatory authority.
How oncologists find therapies fast with the OncoExTra test
- The OncoExTra test reflects a deep understanding of oncologists’ valuable time and priorities, with an actionable report they can quickly review and employ to determine the right therapy for their patients
- Behind the test is a high-tech, high-touch approach that combines ultra-comprehensive sequencing with verification by pathologists
- Integrated full-transcriptome RNA testing identifies fusions for targeted therapy—an essential step that allows oncologists to quickly employ FDA-approved drugs that target fusions2
- Key Biomarker Findings list variants and their associated therapies, including: 1) FDA-approved drugs for a patient’s specific cancer, 2) FDA-approved drugs for other cancers that target the same biomarker, and 3) clinical trials that are actively recruiting patients with a given biomarker